In 1903, Blondlot, who was a well-thought-of French scientist, member of the Academy of Sciences, was experimenting with x-rays as almost everybody was in those days, The effect that he observed was something of this sort. I won't give the whole of it, I'll just give a few outstanding points. He found that if you have a hot wire, a platinum wire, or a Nerns't filament or anything that's heated very hot inside an iron tube and you have a window cut in it and you have a piece of aluminum about 1/8 of an inch thick on it, that some rays come out through that aluminum window. Oh, it can be as much as two or three inches thick and go through aluminum, these rays can, but not through iron. The rays that come out of this little window fall on a faintly illuminated object, so that you can just barely see it. You must sit in a dark room for a long time and he used a calcium sulfide screen which can be illuminated with light and gave out a very faint glow which could be seen in a dark room. Or he used a source of light from a lamp shining through a pinhole and maybe through another pinhole so as to get a faint light on a white surface that was just barely visible.
Now he found that if you turn this lamp on so that these rays that come out of this little aluminum slit would fall on this piece of paper that you are looking at, you could see it much better. Oh, much better, and therefore you could tell whether the rays would go through or not. He said later that a great deal of skill is needed. He said you mustn't ever look at the source. You don't look directly at it. He said that would tire your eyes. Look away from it, and he said pretty soon you'll see it, or you don't see it, depending on whether the N-rays are shining on this piece of paper. In that way, you can detect whether or not the N-rays are acting.
Well, he found that N-rays could be stored up in things. For example, you could take a brick. He found that N-rays would go through black paper and would go through aluminum. So he took some black paper and wrapped a brick up in it and put it out in the street and let the sun shine through the black paper into the brick and then he found that the brick would store N-rays and give off the N-rays even with the black paper on it. He would bring it into the laboratory and you then hold that near the piece of paper that youíre looking at, faintly illuminated, and you can see it much more accurately. Much better, if the N-rays are there, but not if itís too far away. Then, he would have very faint strips of phosphorescent paint and would let a beam of N-rays from two slits come over and he would find exactly where this thing intensified its beam.
Well, you'd think he'd make such experiments as this. To see if with ten bricks you got a stronger effect than you did with one. No, not at all. He didn't get any stronger effect. It didn't do any good to increase the intensity of the light. You had to depend upon whether you could see it or whether you couldn't see it. And there, the N-rays were very important.
Now, a little later, he found that many kinds of things gave off N- rays. A human being gave off N-rays, for example. If someone else came into the room, then you probably could see it. He also found that if someone made a loud noise that would spoil the effect. You had to be silent. Heat, however, increased the effect, radiant heat. Yet that wasn't N-rays itself. N-rays were not heat because heat wouldn't go through aluminum. Now he found a very interesting thing about it was that if you take the brick that's giving off N-rays and hold it close to your head it goes (p.5) through your skull and it allows you to see the paper better. Or you can hold the brick near the paper, thatís all right too.
Now he found that there were some other things that were like negative N-rays. He called them N'-rays. The effect of the N'-rays is to decrease the visibility of a faintly illuminated slit. That works too, but only if the angle of incidence is right. If you look at it tangentially you find that the thing in-creases the intensity when you look at it from this point of view. It decreases if you look at it normally and it increases if you look at it tangentially. All of which is very interesting. And he published many papers on it. One right after the other and other people did too, confirming Blondlotís results. And there were lots of papers published and at one time about half of them that were confirming' the results of Blondlot. You see, N-rays ought to be important because x-rays were known to be important and alpha rays were, and N-rays were somewhere in between so N-rays must be very important. (Laughter)
Well, R. W. Wood heard about these experiments--everybody did more or less. So R. W. Wood went over there and at that time Blondlot had a prism, quite a large prism of aluminum, with a 60o angle and he had a Nernst filament with a little slit about 2 mm wide. There were two slits, 2 mm wide each. This beam fell on the prism and was refracted and he measured the refractive index to three significant figures. He found that it wasn't monochromatic, that there were several different components to the N-rays and he found different refractive indices for each of these components. He could measure three or four different refractive indices each to two or three significant figures, and he was repeating some of these and showing how accurately they were repeatable, showing it to R. W. Wood in this dark room.
Well, after this had gone on for quite a while, and Wood found that he was checking these results very accurately, measuring the position of the little piece of paper within a tenth of a millimeter although the slits were 2 mm wide, and Wood asked him about that. He said, "How? How could you, from just the optics of the thing, with slits two millimeters wide, how do you get a beam so fine that you can detect its position within a tenth of a millimeter?"
Blondlot said, "That's one of the fascinating things about the N-rays. They don't follow the ordinary laws of science that you ordinarily think of." He said, "You have to consider these things all by themselves. They are very interesting, but you have to discover the laws that govern them."
Well, in the meantime, the room being very dark, Wood asked him to repeat some of these measurements which he was only too glad to do. But in the meantime, R. W. Wood put the prism in his pocket and the results checked perfectly with what he had before. (Laughter) Well, Wood rather cruelly published that.(6, 7) And that was the end of Blondlot.
Nobody accounts for by what methods he could reproduce those results to a tenth of a millimeter. Wood said that he seemed to be able to do it but no-body understands that. Nobody understands lots of things. But some of the Germans came out later--Pringsheim was one of them--came out with an extremely interesting story. They had tried to repeat some of Blondlot's experiments and had found this. One of the experiments was to have a very faint source of light on a screen of paper and to make sure that you are seeing the screen of paper you hold your hand up like this and move it back and forth. And if you can see your hand move back and forth then you know it ie illuminated. One of the experiments that Blondlot made was that the experiment was made much better if you had some N-rays falling on the piece of paper. Pringeheim was repeating these in Germany and he found that if you didn't know where the paper was, whether it was here or here (in front or behind your hand), it worked just as well. That is, you could see your hand just as well if you held it back of the paper as if you held it in front of it. Which is the natural thing, because this is a threshold phenomenon. And a threshold phenomenon means that you don't know, you really don't know, whether you are seeing it or not. But if you have your hand there, well, of course, you see your hand because you know your hand's there, and that's just enough to win you over to where you know that you see it. But you know it just as well if the paper happens to be in front of your hand instead of in back of your hand, because you don't know where the paper is but you do know where your hand is. (Laughter)
Well, let's go on. About 1923, there was a whole series of papers by Gurwitech and others. There were hundreds of them published on mitogenetic rays.(8) There are still a few of them being published. I don't know how many of you have ever heard of mitogenetic rays. They are rays that are given off by growing plants, living things, and they were proved, according to Gurwitsch, that they were something that would go through quartz but not through glass. They seemed to be some sort of ultraviolet light.
The way they studied these was this. You had some onion roots- -onions growing in the dark or in the light and the roots will grow straight down. Now if you had another onion root nearby, and this onion root was growing down through a tube or something, going straight down, and another onion root came nearby, this would develop so that there were more cells on one side than the other. One of the tests they had made at first was that this root would bend away. And as it grew this would change in direction which was evidence that something had traveled from one onion root to the other. And if you had a piece of quartz in between it would do it, but if you put glass in between it wouldn't. So this radiation would not go through glass but it would go through quartz.
Well, it started in that way. (p.6) Then everything gave off mitogenetic rays, anything that remotely had anything to do with living things. And then they started to use photoelectric cells to check it and whatever they did they practically always found that if you got the conditions just right, you could just detect it and prove it. But if you looked over those photographic plates that showed this ultraviolet light you found that the amount of light was not much bigger than the natural particles of the photographic plate so that people could have different opinions as to whether it did or didn't show this effect and the result was that less than half of the people who tried to repeat these experiments got any confirmation of it; and so it went. Well, I'll go on before I get too far along.
The characteristics of this Davis-Barnes experiment and the N-rays and the mitogenetic rays, they have things in common. These are cases where there is no dishonesty involved but where people are tricked into false results by a lack of understanding about what human beings can do to themselves in the way of being led astray by subjective effects, wishful thinking or threshold interactions. These are examples of pathological science. These are things that attracted a great deal of attention. Usually hundreds of papers have been published upon them. Sometimes they have lasted for fifteen or twenty years and then they gradually die away.
Now, the characteristic rules are these (see Table I}
Another characteristic thing about them all is that, these observations are near the threshold of visibility of the eyes. Any other sense, I suppose, would work as well. Or many measurements are necessary, many measurements because of very low statistical significance of the results. In the mitogenetic rays particularly it started out by seeing something that was bent. Later on, they would take a hundred onion roots and expose them to something and they would get the average position of all of them to see whether the average had been affected a little bit by an appreciable amount. Or statistical mea-8urements of a very small effect which by taking large numbers were thought to be significant. Now the trouble with that is this. There is a habit with most people, that when measurements of low signifcance are taken they find means of rejecting data. They are right at the threshold value and there are many reasons why you can discard data. Davis and Barnes were doing that right along. If things were doubtful at all why they would discard them or not discard them depending on whether or not they fit the theory. They didn't know that, but that's the way it worked out.
There are claims of great accuracy. Barnes was going to get the Rydberg constant more accurately than the spectroscopists could. Great sensitivity or great specificity, we'll come across that particularly in the Allison effect.
Fantastic theories contrary to experience. In the Bohr theory, the whole idea of an electron being captured by an alpha particle when the alpha particles aren't there just because the waves are there doesn't make a very sensible theory.
Criticisms are met by ad hoc excuses thought up on the spur of the moment. They always had an answer--always.
The ratio of the supporters to the critics rises up somewhere near 50% and then falls gradually to oblivion. The critics can't reproduce the effects. Only the supporters could do that. In the end, nothing was salvaged. Why should there be? There isn't anything there. There never was. Thatís (p.7) characteristic of the effect. Well, I'll go quickly on to some of the other things.
The Allison effect is one of the most extraordinary of all.(9) It started in 1927. There were hundreds of papers published in the American Physical Society, the Physical Review, the Journal of the American Chemical Society--hundreds of papers. Why, they discovered five or six different elements that were listed in the Discoveries of the Year. There were new elements discovered--Alabamine, Virginium, a whole series of elements and isotopes were discovered by Allison.
The effect was very simple. There is the Faraday effect by which
a beam of polarized light passing through a liquid which is in
a magnetic field is rotated--the plane of polarization is
rotated by a longitudinal magnetic field. Now that idea has
been known for a long time and it has a great deal of importance in
connection with light shutters. At any rate, you
can let light through or not depending upon the magnetic field.
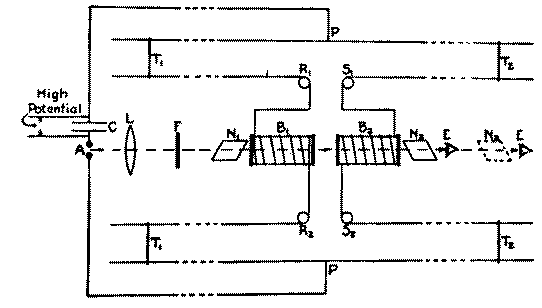
Now the experiment of Allison's was this (Fig. 3). They had a
glass cell and a coil of wire around it (B1, B2) and
you have
wires coming up here, a Lecher system. Here you have a spark
gap, so a flash of light comes through here and goes through a
Nicol prism over here and another one over here, and you adjust
this one with a liquid like water or carbon disulfide or
something like that in the cell so that there was a steady
light over here. If you have a beam of light and you polarize
it and then you turn on a magnetic field, why you see that you
could rotate the plane of polarization. There will be an
increase in the brightness of the light when you put a magnetic
field on here. Now they wanted to find the time delay, how long
it takes. So they had a spark and the same field that produced
the spark induced a current through the coil, and by sliding
this wire along the trolley of the Lecher system, they could
cause a compensating delay. The sensivity of this thing was so
great that they could detect differences of about 3 x 10-10
seconds. By looking in here
they could see these flashes of light, the light from the sparks,
and they tried to decide as they changed the position of this
trolley whether it got brighter or dimmer and they set it for a
minimum, and measured the position of the trolley. They put in
here--in this glass tube--they put a water solution and added
some salt to it. And they found that the time lag was changed,
so that they got a change in the time lag depending upon the
presence of salts.
Fig. 3 Diagram of apparatus and connections. [Copy from F. Allison, Phys. Rev., 30, 66 (1927). Fig. 1].
Now they first found--very quickly--that if you put in a thing like ethyl alcohol that you got one characteristic time lag, and with acetic acid another one, quite different. But if you had ethyl acetate you got the sum of the two. You got two peaks. So that you could analyze ethyl acetate and find the acetic acid and the ethyl alcohol. Then they began to study salt solutions and they found that only the metal elements counted but they didn't act as an ion. That is, all potassium ions weren't the same, but potassium nitrate and potassium chloride and potassium sulfate all had quite characteristic different points, that were a characteristic of the compound. It was only the positive ion that counted and yet the negative ions had a modifying effect. But you couldn't detect the negative ions directly.
Now they began to see how sensitive it was. Well, they found that any intensity more than about 10-8 molar solution would always produce the maximum effect, and you'd think that that would be kind of discouraging from the analytical point of view, but no, not at all. And you could make quantitative measurements to about three significant figures by diluting the solutions down to a point where the effect disappeared. Apparently, it disappeared quite sharply when you got down to about 10-8 or 3. 42 x 10-8 in concentration, or something of that sort and then the effect would disappear. Otherwise, you would get it, so that you could detect the limit within this extraordinary degree of accuracy.
Well, they found that things were entirely different, even in these very dilute solutions, in sodium nitrate from what it was with sodium chloride. Nevertheless, it was a characteristic which depended upon the compound even though the compound was disassociated into ions at those concentrations. That didn't make any difference but it was fact that was experimentally proven. They then went on to find that the isotopes all stick right out like sore thumbs with great regularity. In the case of lead, they found sixteen isotopes. These isotopes were quite regularly spaced so that you could get 16 different positions and you could assign numbers to those so that you can identify them and tell which they are. Unfortunately, you couldn't get the concentrations quantitatively, even the dilution method didn't work quite right because they weren't all equally sensitive. You could get them relatively but only approximately. Well, it became important as a means of detecting elements that hadn't yet been discovered, like Alabamine and elements that are now known, and filling out the periodic table. (p.8) All the elements in the periodic table were filled out that way and published.
But a little later, in 1945 or 46, I was at the University of California. Owen Latimer who is now Head of the Chemistry Department there--not Owen Latimer, Wendell Latimer--had had a bet with G. N. Lewis (in 1932). He said, "There's something funny about this Allison effect, how they can detect isotopes." He had known somebody who had been down with Allison and who had been very much impressed by the effect and he said to Lewis, "I think I'll go down and see Allison, to Alabama, and see what there is in it. I'd like to use some of these methods."
Now people had begun to talk about spectroscopic evidence that there might be traces of hydrogen of atomic weight three. It wasn't spoken of as tritium at that time but hydrogen of atomic weight three that might exist in small amounts. There was a little spectroscopic evidence for it and Latimer said, "Well, this might be a way of finding it. I'd like to be able to find it." So he went and spent three weeks at Alabama with Allison and before he went he talked it over with G. N. Lewis about what he thought the prospects were and Lewis said, "I'll bet you ten dollars you'll find that there's nothing in it.Ē And so they had this bet on. He went down there and he came back. He set up the apparatus and made it work so well that G. N. Lewis paid him the ten dollars. (Laughter) He then discovered tritium and he published an article in the Physical Review.(10) Just a little short note saying that using Allison's method he had detected the isotope of hydrogen of atomic weight three. And he made some sort of estimate as to its concentration.
Well, nothing more was heard about it. I saw him then, seven or eight years after that. I had written these things up before, about this Allison effect, and I told him about this point of view and how the Allison effect fits all these characteristics. Well, I know at that time at one of the meetings of the American Chemical Society there was great discussion as to whether to accept papers on the Allison effect. There they decided: No, they would not accept any more papers on the Allison effect, and I guess the Physical Review did too. At any rate, the American Chemical Society decided that they would not accept any more manuscripts on the Allison effect. However, after they had adopted that as a firm policy, they did accept one more a year or two later because here was a case where all the people in the faculty here had chosen twenty or thirty different solutions that they had made up and they had labeled them all secretly and they had taken every precaution to make sure that nobody knew what was in these solutions, and they had given them to Allison and he had used his method on them and he had gotten them all right, although many of them were at concentrations of 10-6 and so on, molar. That was sufficiently definite -- good experimental methods -- and it was accepted for publication by the American Chemical Society but that was the last.(11) You'd think that would be the beginning, not the end.
Anyway, Latimer said, "You know, I don't know what was wrong with me at that time,' He said, "After I published that paper I never could repeat the experiments again. I haven't the least idea why." "But," he said, "Those results were wonderful, I showed them to G. N. Lewis and we both agreed that it was all right. They were clean cut. I checked myself every way I knew how to. I don't know what else I could have done, but later on I just couldn't ever do it again."
I don't know what it is. Thatís the kind of thing that happens in all of these. All the people who had anything to do with these things find that when you get through with them--you can't account for Bergen Davis saying that they didnít calculate those things from the Bohr theory, that they were found by empirical methods without any idea of the theory. Barnes made the experiments, brought them in to Davis, and Davis calculated them up and discovered all of a sudden that they fit the Bohr theory. He said Barnes didn't have anything to do with that. Well, take it or leave it, how did he do it? It's up to you to decide. I can't account for it. All I know is that there was nothing salvaged at the end, and therefore none of it was ever right, and Barnes never did see a peak. You can't have a thing halfway right.